Fda Drug Regulations
FDA regulations are based on the laws set forth in the Tobacco Control Act and the Food Drug and Cosmetic Act FDC Act. Owing to the trying times of COVID-19 pandemic it is important to track the movement of products specifically drugs.
 Facts That Everyone Should Know About Fda Regulation Compliance4all
Facts That Everyone Should Know About Fda Regulation Compliance4all
The FDA Food and Drug Administration is a government agency responsible for upholding and protecting the safety and health of the United States general public.
Fda drug regulations. FDA QR Code-based regulations. FDA ensures the quality of drug products by carefully monitoring drug manufacturers compliance with its Current Good Manufacturing Practice CGMP regulations. The content of the prescription drug.
Regulations have a way of expanding far beyond the size of the enabling law. A FDA may approve a sponsors marketing application for a designated orphan drug for use in the rare disease or condition for which the drug was designated or for select indications or uses. The CGMP regulations for drugs.
Here are FDA regulations which mandate a QR Code. The FDA regulates more than US24 trillion worth of consumer goods about 25 of consumer expenditures in the United States. Any labelling information submitted electronically must be completed in accordance with the Structured Product Labelling SPL standard based on extensible markup XML language.
According to FDA guidelines for submitting documents for packaging for human drugs and biological the followingare required. 1 For human prescription drugs that are subject to section 505 of the Federal Food Drug and Cosmetic Act or section 351 of the Public Health Service Act. For example the Food Drug and Cosmetic Act consisted of a mere 19 pages.
The FDA has strict requirement for drugs labelling and health claim substantiation. The History of Drug Regulation Federal regulation of drugs emerged as early as 1848 under a law that addressed only imported drugs. Hence it becomes important to check the authenticity of the product.
DrugsFDA includes information about drugs including biological products approved for human use in the United States see FAQ but does not include information about FDA-approved products. A FDA inspection. In 1973 the FDA published regulations to take effect in 1975 expanding its control over supplements by declaring that any dietary supplement that it considered to lack nutritional usefulness was a drug and thus under the FDAs control.
Congress and regulations established by the Agency to protect the consumers health safety and pocketbook. This includes 466 billion in food sales 275 billion in drugs 60 billion in cosmetics and 18 billion in vitamin supplements. Package must maintain standards identity strength quality purity for intended shelflife Full informationneeded Type of containerclosure Suitabilityforintendeduse Submissionof packaginginformationdate.
A sponsor shall upon request from any properly authorized officer or employee of the Food and Drug Administration at reasonable times permit such. In 1905 the American Medical Association launched a private. For example the United States Food and Drug Administration US FDA has the rule-making responsibility for the Food Drug and Cosmetic Act of 1938 in the United States US.
The mission of FDA is to enforce laws enacted by the US. The FDA promotes these goals through the implementation of standards regarding food medications medical devices cosmetics dietary supplements and tobacco. FDA regulations are also.
QR Code on export certificates. FDA considers the requirements in 21 CFR Parts 210 and 211 to. REQUIREMENTS FOR FOREIGN AND DOMESTIC ESTABLISHMENT REGISTRATION AND LISTING FOR HUMAN DRUGS INCLUDING DRUGS THAT ARE REGULATED UNDER A BIOLOGICS LICENSE APPLICATION AND ANIMAL DRUGS AND THE.
As required by law the Food and Drug Administration publishes regulations in the Federal Register the federal governments official publication for notifying the. And to ensure this FDA has added a new QR Code structure for food exports. This document guide is intended to provide guidance regarding good manufacturing practice GMP for the manufacturing of active pharmaceutical ingredients API under an appropriate system for managing quality.
FDA Memorandum Circular No2021-0001 Extension of validity of License to Operate LTO and other market authorizations granted to Veterinary Establishments Drugs Biologicals and Products transferred from the Bureau of Animal Industry BAI to the Food nd Drug Administration FDA Published on 7.
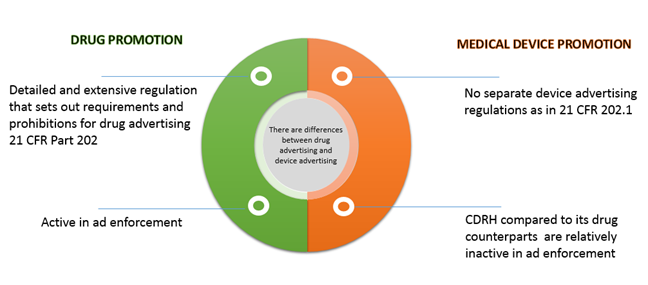
 Understanding The Fda Regulations Governing Advertising And Promotion Of Drugs And Medical Devices
Understanding The Fda Regulations Governing Advertising And Promotion Of Drugs And Medical Devices
 U S Food And Drug Administration
U S Food And Drug Administration
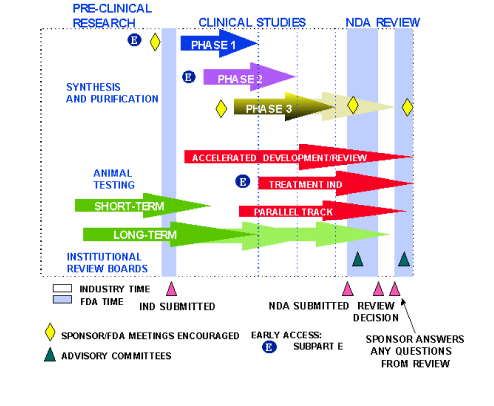
Fda Update The Fda S New Drug Approval Process Development Premarket Applications
Fda Update The Fda S New Drug Approval Process Development Premarket Applications
 Fda Regulation Of Prescription Drugs Nejm
Fda Regulation Of Prescription Drugs Nejm
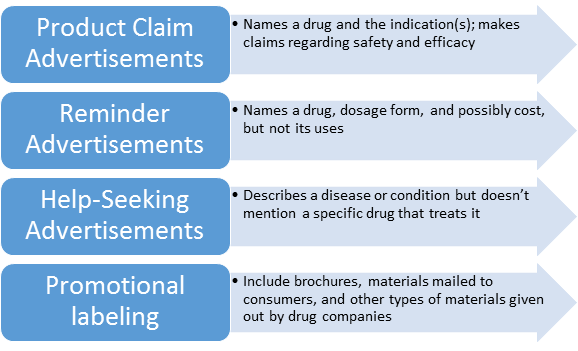 Understanding The Fda Regulations Governing Advertising And Promotion Of Drugs And Medical Devices
Understanding The Fda Regulations Governing Advertising And Promotion Of Drugs And Medical Devices
 How To Get Fda Approval Registrar
How To Get Fda Approval Registrar
 Fda Regulatory Policy Friends Of Cancer Research
Fda Regulatory Policy Friends Of Cancer Research
 Fda Drug Approval Regulations Safety
Fda Drug Approval Regulations Safety
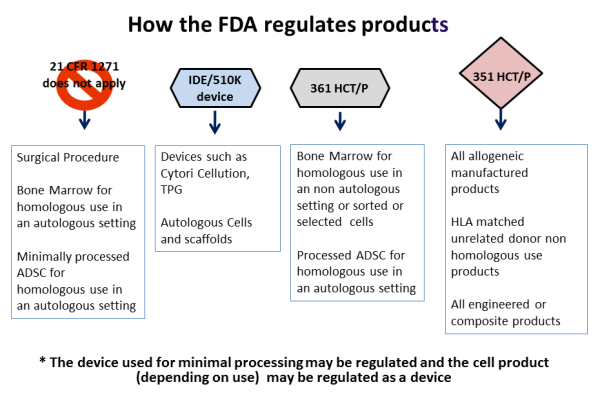
 Regulation Of Food And Dietary Supplements By The U S Food And Drug Administration Wikipedia
Regulation Of Food And Dietary Supplements By The U S Food And Drug Administration Wikipedia
 An Overview Of Fda Requirements For Otc Drugs Over The Counter Products And Understanding Fda Regulation For Otc Drugs Meet All The Fda Requirements Quick And Easy Excellent Customer
An Overview Of Fda Requirements For Otc Drugs Over The Counter Products And Understanding Fda Regulation For Otc Drugs Meet All The Fda Requirements Quick And Easy Excellent Customer

Comments
Post a Comment